Dosing Overview
Images are not of actual patients
ULTOMIRIS® provides adult patients with the freedom of predictable, once-every-8-week maintenance dosing starting 2 weeks after an initial loading dose1,2
Dosing Regimen
The ULTOMIRIS dosing regimen offers predictable dosing which allows patients more time between infusions to do what they love
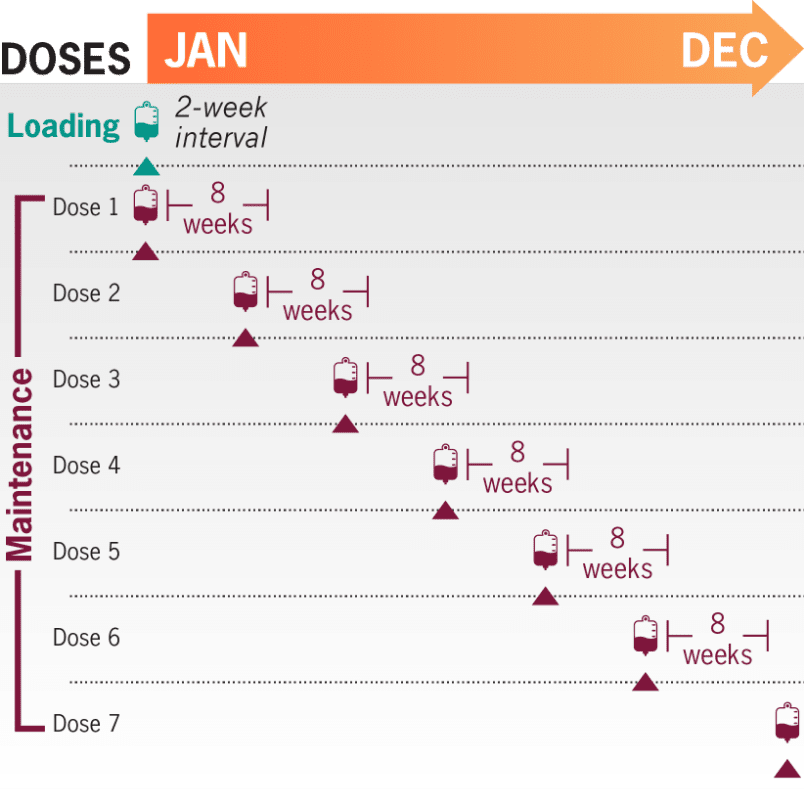
6-7 maintenance infusions per year1
Each infusion typically lasts less than 1 hour for the majority of patients.1,a
- Patients are monitored for at least 1 hour after infusions for signs or symptoms of an infusion-related reaction1
- If an adverse reaction occurs during the administration of ULTOMIRIS (ravulizumab-cwvz), the infusion may be slowed or stopped at the discretion of the physician1
- The recommended weight-based dosing regimen in adult patients with generalized myasthenia gravis (gMG) (≥40 kg [88 lb]) consists of a loading dose followed 2 weeks later by the start of maintenance dosing every 8 weeks1
- The dosing schedule is allowed to occasionally vary within 7 days of the scheduled infusion day (except for the first maintenance dose of ULTOMIRIS), but subsequent doses should be administered according to the original schedule1
- Following a missed intravenous ULTOMIRIS dose, the patient should contact their healthcare provider immediately1
aMinimum infusion time for ULTOMIRIS 100 mg/mL maintenance doses ranges from 30 minutes to less than 1 hour, depending on body weight.1
WATCH: gMG expert discusses ULTOMIRIS dosing
Watch Deena Rodney, APRN, provide a deep dive on the predictable ULTOMIRIS dosing regimen and administration.
Download: Dosing and Administration Guide
Infusion Locator
Looking for an infusion location for your patient?

Jesse has received compensation from Alexion Pharmaceuticals, Inc. It is not known if ULTOMIRIS® is safe and effective for the treatment of gMG in children.
Location
If you would like to be added to the list of infusion locations, email us at: UltomirisInfusionLocator@astrazeneca.com. In your email, please provide: Name of Practice, Street Address, City, State, ZIP Code, Phone Number, and Website URL (optional).

Jesse has received compensation from Alexion Pharmaceuticals, Inc. It is not known if ULTOMIRIS® is safe and effective for the treatment of gMG in children.
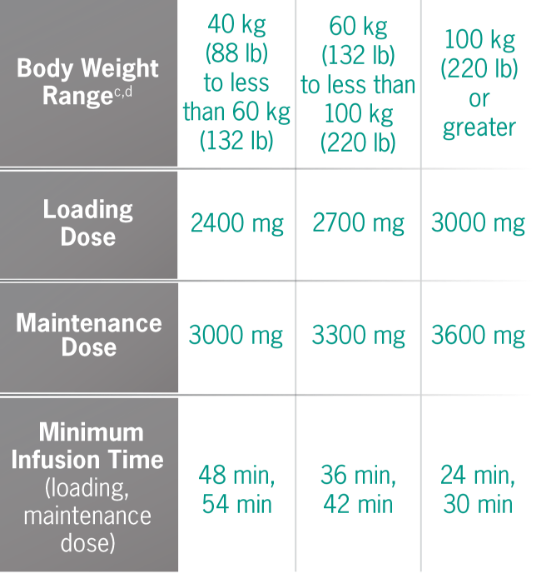
Doses and infusion times for ULTOMIRIS 100 mg/mL1
bBody weight at time of treatment.1
cApproximate weight in pounds was calculated using standard weight conversion of 1 kg=2.205 lb.
If an adverse reaction occurs during the administration of ULTOMIRIS, the infusion may be slowed or stopped at the discretion of the physician. Monitor the patient for at least 1 hour following completion of the infusion for signs or symptoms of an infusion-related reaction.1
ULTOMIRIS Dosing Calculator
This calculator tool is for reference only. See the full dosing chart above and the recommended weight-based dosing regimen for gMG in section 2.6 of the ULTOMIRIS Prescribing Information.
Units
Loading dose (mg)
Maintenance dose (mg)
100 mg/mL formulation loading dose vials
100 mg/mL formulation maintenance dose vials
Weight-based dosing
The recommended dosage of ULTOMIRIS in adult patients with gMG weighing 40 kg or greater is based on the patient’s body weight.1
IV line flushing
The IV infusion set tubing should be flushed at the end of the infusion to help ensure the full dose of ULTOMIRIS is administered. This is important for all patients, especially due to the weight-based dosing and small volume (<100 mL) of ULTOMIRIS 100 mg/mL.1
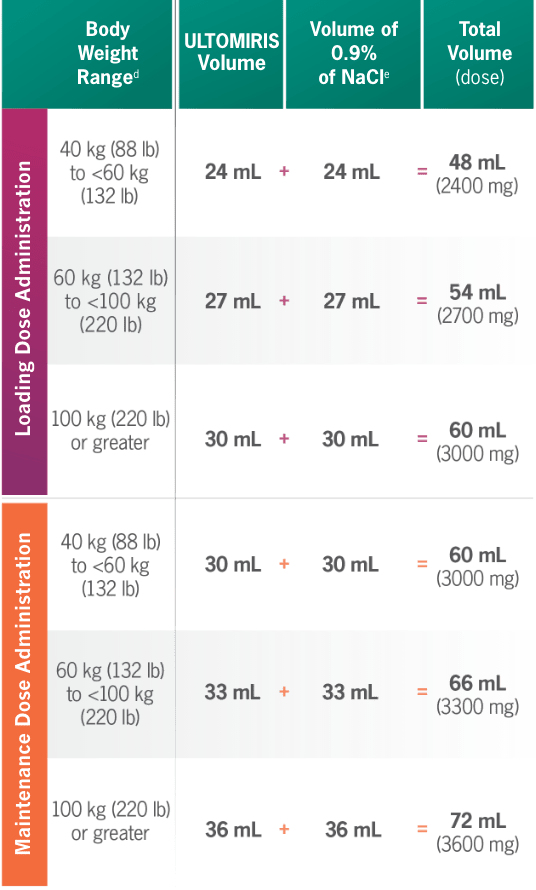
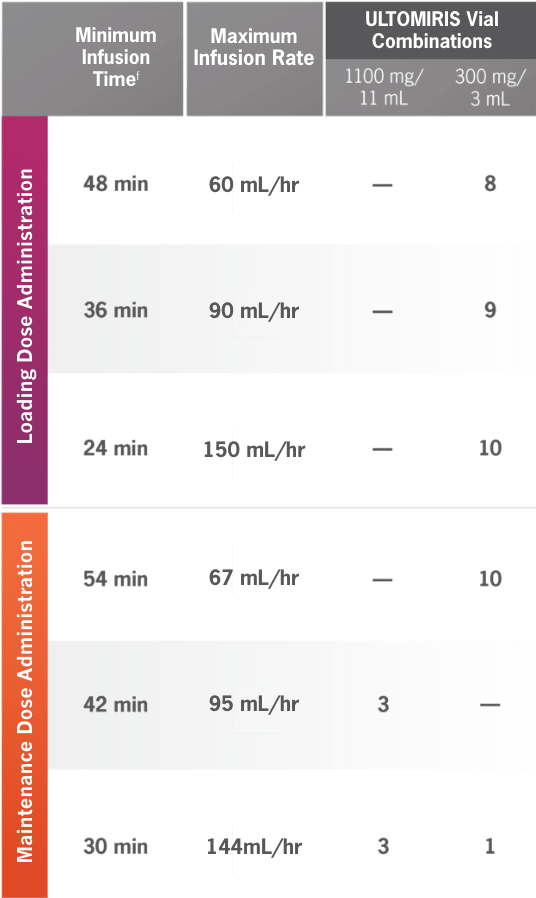
ULTOMIRIS 100 mg/mL dosing at a glance1
| Body Weight Ranged |
ULTOMIRIS Volume | Volume of |
|
Minimum |
Maximum |
ULTOMIRIS Vial
Combinations
1100 mg/
11 ml 300 mg/ 3 ml |
||
|---|---|---|---|---|---|---|---|---|
|
Loading Dose Administration
|
40 kg (88 lb) to <60 kg (132 lb) |
24 mL + | 24 mL= | 48 mL (2400 mg) | 48 min | 60 mL /hr | — | 8 |
| 60 kg (132 lb) to <100 kg (220 lb) |
27 mL+ | 27 mL = | 54 mL (2700 mg) | 36 min | 90 mL /hr | — | 9 | |
| 100 kg (220 lb) or greater |
30 mL+ | 30 mL = | 60 mL (3000 mg) | 24 min | 150 mL/hr | — | 10 | |
|
Maintenance Dose Administration
|
40 kg (88 lb) to <60 kg (132 lb) |
30 mL+ | 30 mL = | 60 mL (3000 mg) | 54 min | 67 mL/hr | — | 10 |
| 60 kg (132 lb) to <100 kg (220 lb) |
33 mL+ | 33 mL = | 66 mL (3300 mg) | 42 min | 95 mL/hr | 3 | — | |
| 100 kg (220 lb) or greater |
36 mL+ | 36 mL = | 72 mL (3600 mg) | 30 min | 144 mL/hr | 3 | 1 | |
dBody weight at time of treatment.1
eDilute ULTOMIRIS only using 0.9% Sodium Chloride Injection, USP.1
fMinimum infusion time for ULTOMIRIS 100 mg/mL maintenance doses ranges from 30 minutes to less than 1 hour, depending on body weight.1
Supplemental dosing of ULTOMIRIS after PE, PP, or IVIg1
Concomitant use of ULTOMIRIS with PE, PP, or IVIg treatment can reduce serum ULTOMIRIS concentrations and requires a supplemental dose of ULTOMIRIS.
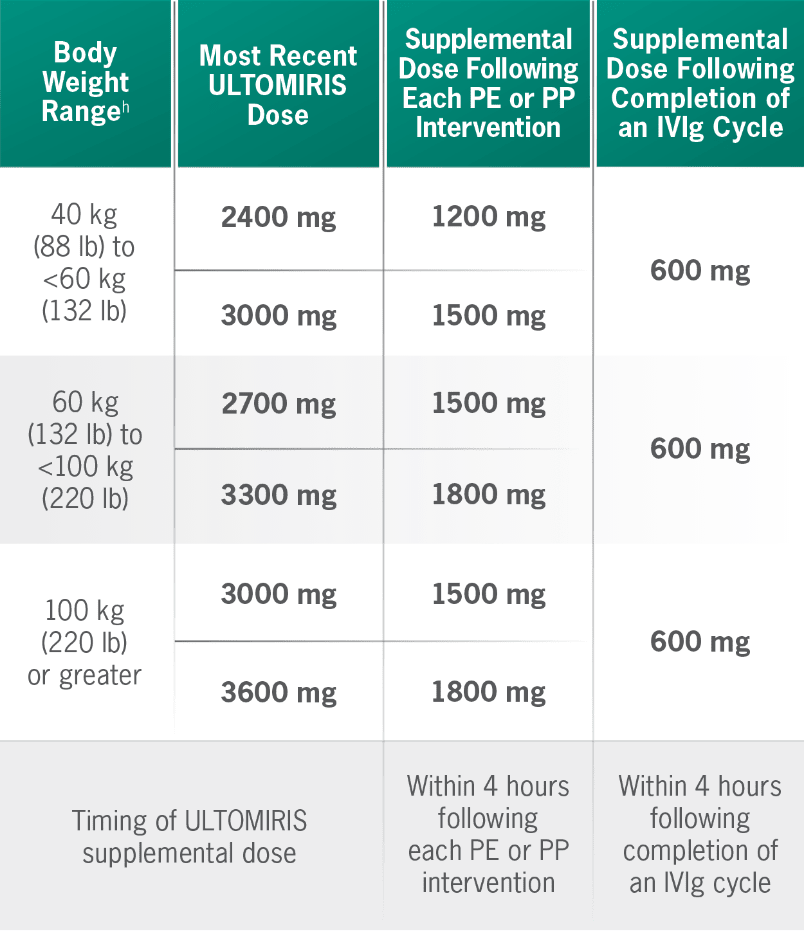
gBody weight at time of treatment.1
Neonatal Fc receptor (FcRn) blockers
Concomitant use of ULTOMIRIS with FcRn blockers (eg, efgartigimod) may lower systemic exposures and reduce the effectiveness of ULTOMIRIS. Closely monitor for reduced effectiveness.1
With a predictable infusion schedule, ULTOMIRIS increases freedom between doses for adult patients with gMG who are anti-AChR antibody positive. ULTOMIRIS offers the only once-every-8-week maintenance dosing schedule starting 2 weeks after an initial dose1
AChR, acetylcholine receptor; gMG, generalized myasthenia gravis; IVIg, intravenous immunoglobulin; NaCl, sodium chloride; PE, plasma exchange; PP, plasmapheresis; USP, United States Pharmacopeia.

Mike has received compensation from Alexion Pharmaceuticals, Inc. and has a relative who works for Alexion.